Collaborative Projects
KETONe bodies In diseases of the Cardiovascular System (KETONICS)
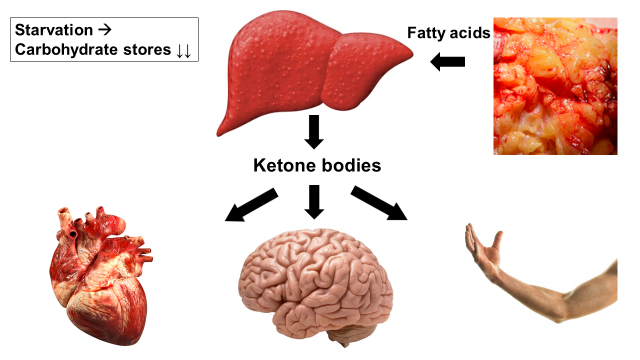
As we have demonstrated that the ketone body 3-hydroxybutyrate (3-OHB) has pronounced beneficial effect on the heart, the overall aim of KETONICS is to translate studies on ketone metabolism to the clinical treatment of patients with cardiovascular disease, i.e. heart failure, diabetes and hypertension. Basal cardiac and vascular physiological mechanisms underlying the effect of the ketone body 3-OHB are studied in pigs, rodents, zebrafish and cellular models. Pathophysiological mechanisms are further explored in patients and animals by invasive and non-invasive measurements of cardiovascular function and metabolism using state-of-the-art methods. These include cardiac magnetic resonance imaging (CMRI), positron emission tomography, hyperpolarized [1-13C]-pyruvate CMRI, and studies of circulating hormones and metabolites and vasculature. Our findings will be translated into clinical, randomized studies. The signaling function of ketone bodies will also be examined. Finally, new approaches to increase circulating 3-OHB will be explored through synthesis of novel orally available pro-ketogenic compounds as well as by high throughput screening of known pharmaceuticals and novel peptide hormones for their potential ketogenic effects initially in vitro.
Aarhus University Cardiovascular research group:
- Professor Henrik Wiggers, Department of Cardiology, Aarhus University Hospital
- Professsor Niels Møller, Dept. of Endocrinology and Metabolism, Aarhus University Hospital
- Ass. professor Lars Gormsen, Department of Nuclear Medicine, Aarhus University Hospital
- Postdoc, MD, PhD Roni Nielsen, Department of Cardiology, Aarhus University Hospital
- Ass. professor, Christoffer Laustsen, The MR research center, Aarhus University
- Professor Mogens Johannsen, Dept. for Toxicology and Drug Analysis, Dept. of Forensic Medicine
- Professor Christian Aalkjær, Department of Biomedicine, Aarhus University
- Professor Claus Oxvig, Department of Molecular Biology and Genetics, Aarhus University
- Professor Hans Erik Bøtker, Department of Cardiology, Aarhus University Hospital
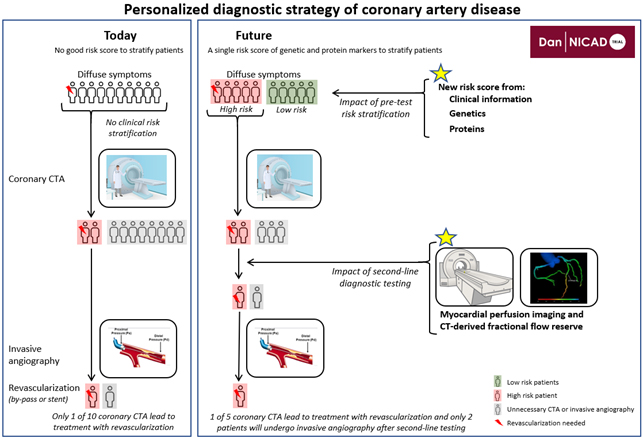
Personalized diagnostic strategy of coronary artery disease
Severe coronary artery disease (CAD) can develop despite the absence of traditional risk factors or be avoided entirely despite having multiple risk factors. Hence, genetic variations and environmental contributors are believed to strongly influence the formation of coronary atherosclerosis. Because symptoms of CAD are often uncharacteristic, millions of patients worldwide undergo investigations to rule out CAD without having the disease - in many studies up to 80 % of the referred patients.
Currently there is a gap in the evidence regarding which diagnostic strategy to use in these millions of patients referred for CAD rule-out around the world. Hence, the overall aim of the Dan-NICAD trial is to develop a diagnostic strategy with personalised risk assessment followed by an optimal non-invasive diagnostic strategy in patients with symptoms suggestive of CAD.
The regional multicentre trial, Dan-NICAD, has today included >3,000 patients with symptoms of CAD in the Dan-NICAD 1 and the ongoing Dan-NICAD 2 trial. Patients is investigated with CTA, myocardial perfusion imaging and invasive angiography. Whole genome sequencing and proteomics using OLINK assays has been performed.
The perspective of a personalized risk assessment model based on risk factors, genetics, proteins and relevant diagnostic testing is likely to have a major impact on patient management and health resource utilization in the future. The perspective is to drive the diagnosis of CAD into the era of personalized medicine.
Primary investigators:
- Ass. Professor, Morten Bøttcher, Department of Cardiology, Hospital Unit West
- Ass. Professor, Simon Winther, Department of Cardiology, Hospital Unit West
- Ass. Professor, Mette Nyegaard, Department of Biomedicine, Aarhus University
National collaborative and research group:
- PhD Morten K. Christiansen, Department of Cardiology, Aarhus University Hospital
- Professor Hans Erik Bøtker, Department of Cardiology, Aarhus University Hospital
- Ass. professor Evald Høj Christiansen, Department of Cardiology, Aarhus University Hospital
- Ass. professor Lars Gormsen, Department of Nuclear Medicine, Aarhus University Hospital
- Ass. professor Lars Frost, Department of Cardiology, Hospital Central Unit
- Chief physician, Hanne Maare Søndergaard, Department of Cardiology, Hospital Central Unit
- Chief physician, June Eilersen, Department of Nuclear Medicine, Hospital Unit West
- Chief physician, Christin Rosendahl Graff Isaksen Department of Radiology, Hospital Central Unit
International collaborative
- Professor Juhani Knuuti, PET center, Turku University Hospital, Finland
- Professor Steffen E. Petersen, Barts Heart Centre, London, UK
- Professor Pamela S Douglas, Research in Cardiovascular Disease, Duke University, US
- deCODE genetics, Iceland
PhD projects/student:
- Louise Nissen, MD
- Peter L. Møller, MSc (ongoing)
- Laust D. Rasmussen, MD (ongoing)
- Jelmer S. Westre, MD (ongoing)
- Salma R. K. Abdulzahra, MD (ongoing)